Product features and clinical data
Learn more about the characteristics of Belotero Balance (+) that set it apart from other hyaluronic acid (HA) fillers and make it an optimal choice for your patients.
AN HA FILLER DESIGNED TO FLEX
Rheologic properties—such as cohesivity, elasticity, and viscosity—are used as a scientific rationale when selecting the appropriate filler for specific clinical applications. Learn more about the clinical significance of these properties, and then understand how the rheologic profile of Belotero Balance (+) contributes to its ability to achieve results with a smooth and natural feel.1-5
Cohesivity
Definition: Capacity of a material to not dissociate, because of the intrinsic affinity of the constituent molecules for each other1
Clinical significance: Ability to resist heterogeneous distribution after introduction into the tissue
Elasticity
Definition: Ability of a gel to resist deformation when pressure is applied9
Clinical significance: Capacity to support tissue
Viscosity
Definition: Ability of a gel (in the fluid phase) to resist shearing forces1,4,9
Clinical significance: Ability to spread and integrate into tissues
The Belotero Balance (+) formula
High cohesivity + balanced elasticity & viscosity1
These attributes of Belotero Balance (+) give it a soft, spreading quality with a tendency to flow and homogeneously integrate into tissue, as well as the ability to maintain its gel structure when mechanically stressed.1,3,5 This is important for treating etched-in lines, which tend to be present in very mobile and highly visible facial areas with little room for post-injection swelling.1,4
Belotero Balance (+) has HIGH cohesivity
In HA fillers, cohesivity is a deciding factor to consider
Evaluating cohesivity helps you better understand and leverage distribution patterns and clinical behaviors of different HA fillers.
Belotero Balance (+) has an ideal rheologic profile, including high cohesivity, that helps1,4:
- Maintain gel integrity
- Contribute to tissue support
- Inject and spread evenly
Overall, Belotero Balance (+) was found to be the most cohesive among FDA-approved HA fillers evaluated with the Gavard-Sundaram Cohesivity scale10*†
Cohesivity is difficult to measure. The Gavard-Sundaram Cohesivity Scale was developed as a standardized protocol for measuring HA cohesivity and evaluating HA fillers. To measure cohesivity levels, each filler was10:
- Dyed blue
- Injected into a beaker of water
- Evaluated at various time intervals for its ability not to dissociate (ie, to maintain its shape in the water)
* Belotero Balance(+) was compared to Juvéderm Ultra, Juvéderm Ultra Plus, Juvéderm Voluma, Restylane, Restylane Sub Q.
† Clinical implications of in vitro data comparison have not been studied.
Standardized Photographic Cohesivity Scale
To create this scale, the cohesivity assay was performed on 10 types of HA fillers, during which a total of 90 photographs (3 assays x 3 time points x 10 specimens) were taken and reviewed. Through this process, 5 principal patterns of gel behavior were identified and then used to develop a 5-point visual reference scale.10
Validation of the Cohesivity Assay
A panel of 6 plastic surgeons and dermatologists served as raters to determine the reliability, sensitivity, and repeatability of the cohesivity assay.10
- Panelists had no prior training or exposure to any assay images
- Reliability, sensitivity, and repeatability of the cohesivity assay were determined by viewing 60 photos (2 assays x 3 time points x 10 specimens)
- Good inter-rater consistency and repeatability were observed for most images
| Cohesivity score | Kappa | SE | 95% CI | P |
|---|---|---|---|---|
| 1 | 0.72126 | 0.033333 | 0.65593-0.7866 | <0.0001 |
| 2 | 0.27885 | 0.033333 | 0.21351-0.34418 | <0.0001 |
| 3 | 0.65666 | 0.033333 | 0.59133-0.722 | <0.0001 |
| 4 | 0.61325 | 0.033333 | 0.54791-0.67858 | <0.0001 |
| 5 | 0.73034 | 0.033333 | 0.665-0.79567 | <0.0001 |
| Overall weighted kappa | 0.62798 | 0.017488 | 0.5937-0.66225 | <0.0001 |
| Kendall coefficient of concordance | 0.94752 | <0.0001 |
Compare the cohesivity of different HA fillers10
Belotero Balance®
Cohesive polydensified matrix
Cohesivity score*: 5.0
(fully cohesive)
Juvéderm® Ultra XC
Hylacross™
Cohesivity score*: 4.1 at 95 secs
(mostly cohesive)
Belotero Balance®
Cohesive polydensified matrix
Cohesivity score*: 5.0
(fully cohesive)
Juvéderm® Ultra PLUS XC
Hylacross™
Cohesivity score*: 4.1 at 95 secs
(mostly cohesive)
Belotero Balance®
Cohesive polydensified matrix
Cohesivity score*: 5.0
(fully cohesive)
Juvéderm® Voluma® XC
Vycross™
Cohesivity score*: 1.3 at 95 secs
(mostly dispersed)
Belotero Balance®
Cohesive polydensified matrix
Cohesivity score*: 5.0
(fully cohesive)
RESTYLANE®
Non–animal stabilized
Cohesivity score*: 1.0 at 95 secs
(mostly dispersed)
Evaluation of HA fillers with the Gavard-Sundaram Cohesivity Scale after extrusion11
In an evaluation of HA fillers with the Gavard-Sundaram Cohesivity Scale after extrusion:
| Product | Time points of analysis (seconds) | Cohesivity score |
|---|---|---|
| Restylane Lyft | 15 | 1 |
| 70 | 1 | |
| 95 | 1 | |
| Restylane-L | 15 | 2 |
| 70 | 1 | |
| 95 | 1 | |
| Juvéderm Voluma | 15 | 3 |
| 70 | 2 | |
| 95 | 2 | |
| Juvéderm Volbella | 15 | 3 |
| 70 | 2 | |
| 95 | 2 | |
| Restylane Silk | 15 | 5 |
| 70 | 3 | |
| 95 | 3 | |
| Juvéderm Ultra Plus XC | 15 | 5 |
| 70 | 4 | |
| 95 | 4 | |
| Juvéderm Ultra XC | 15 | 5 |
| 70 | 4 | |
| 95 | 4 | |
| Belotero Balance | 15 | 5 |
| 70 | 5 | |
| 95 | 5 |
*No significant difference in cohesivity score before and after extrusion through a needle. Time points of analysis: 15, 70, and 95 seconds.10,11
Belotero Balance (+) has LOW elasticity
Compare the elasticity of 8 HA fillers
Elastic modulus (G’) after needle extrusion* at testing frequency (0.7 Hz) after extrusion12
In an evaluation of HA fillers with the Gavard-Sundaram Cohesivity Scale:
| Product | G’ (0.7 Hz), Pa (measure of material stiffness) |
|---|---|
| Restylane Lyft | 747 |
| Restylane-L | 732 |
| Restylane Silk | 517 |
| Juvéderm Voluma | 305 |
| Juvéderm Volbella | 213 |
| Juvéderm Ultra Plus XC | 146 |
| Juvéderm Ultra XC | 110 |
| Belotero Balance | 41 |
*No significant difference in cohesivity score before and after extrusion through a needle.
Belotero Balance (+) has LOW viscosity
Compare the viscosity of 8 HA fillers
Complex viscosity (η*) after needle extrusion* at testing frequency (0.7 Hz) after extrusion12
In an evaluation of HA fillers with the Gavard-Sundaram Cohesivity Scale:
| Product | η* (0.7 Hz), Pa.s (measure of ability to spread) |
|---|---|
| Restylane Lyft | 191 |
| Restylane-L | 188 |
| Restylane Silk | 136 |
| Juvéderm Voluma | 77 |
| Juvéderm Volbella | 54 |
| Juvéderm Ultra Plus XC | 38 |
| Juvéderm Ultra XC | 29 |
| Belotero Balance | 10 |
*No significant difference in viscosity score before and after extrusion through a needle.
CROSS-LINKING TECHNOLOGY SUPPORTS OPTIMAL TISSUE INTEGRATION
PHASE 1
HA in its original form
(single chains, random coil structure)1
PHASE 2
Linearization of the individual HA
chains; the random coil structure
untangles1
PHASE 3
First HA cross-linking process
with butanediol diglycidyl ether (BDDE);
a cellular, monophasic gel emerges1
PHASE 4
Expansion of the gel that was created
in the cross-linking process14
PHASE 5
A second application of HA
links the residual BDDE to the fibers; the gel results in a cohesive polydensified
matrix14
Different cross-linking technologies and the degree and type of cross-linking result in HA dermal fillers with various rheologic profiles and unique biophysical characteristics.
Belotero Balance (+) is manufactured to have different proportions of HA cross-linking density to provide the cohesivity required for seamless, natural tissue integration in harmony with facial movements.13 Additionally, the dynamic cross-linking manufacturing processes of Belotero Balance (+) create1:
- A cohesive and homogeneous gel
- Zones of greater or lesser cross-linking density
- Low elasticity and viscosity and high tan delta
Compare Belotero Balance (+) tissue integration with other HA fillers
Belotero Balance (+) demonstrates a homogenous pattern of distribution and optimal tissue integration3,5
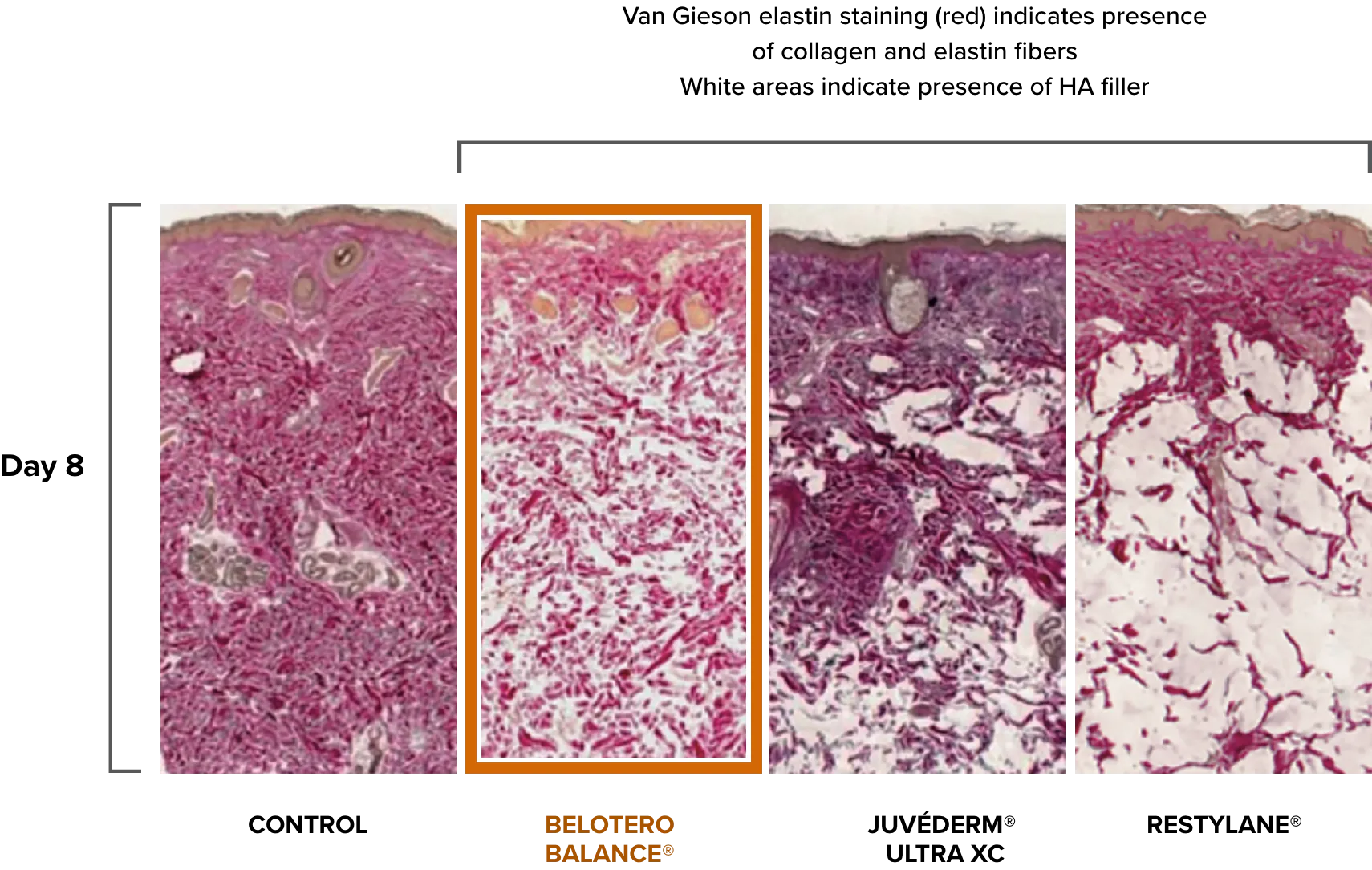
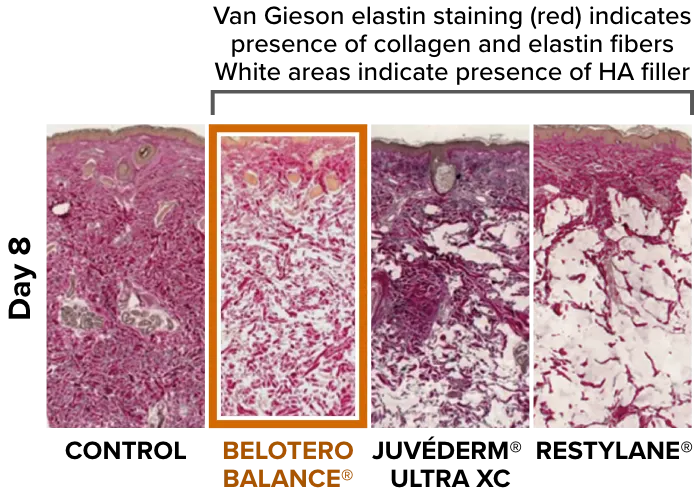
Adapted from Tran C, et al. Dermatology. 2014;228(1):47-54.
Van Gieson elastin staining comparison after 8 days of control, Belotero Balance, Juvéderm Ultra XC, and Restylane, demonstrating more homogenous tissue integration with Belotero Balance (+).3
CLINICAL TRIAL RESULTS SUPPORT BELOTERO BALANCE (+) EFFICACY AND DURATION
Belotero Balance (+) has been evaluated in a long-term clinical study in patients treated with Belotero Balance (+) in one nasolabial fold (NLF) and bovine collagen in the other NLF.7
Results last 6 months or longer7,15
- Primary endpoint: Mean change from baseline score of each NLF 12 weeks after last injection
- Belotero Balance (+) showed a significantly greater reduction in NLF compared to bovine collagen at weeks 8 (P=0.009), 12 (P<0.001), and 24 (P<0.001)
- A majority of patients did not need a repeat injection for at least 48 weeks15
Additional study details7,15
- All patients were given Belotero Balance in one NLF and bovine collagen in the
other NLF - There was an option for touch-up at 2 weeks
- Patients returned every 2 weeks through 24 weeks for evaluation
- Results were as assessed by both the treating physician and blinded evaluator on the Wrinkle Severity Rating Scale
- Open-label extension allowed for treatment of Belotero Balance (+) in both NLFs that lasted from 24 to 96 weeks after the initial visit. A touch-up was allowed at 32 weeks
Mean Change from baseline of Wrinkle Severity Rating Scale at Week 2415
Mean change from baseline of Wrinkle Severity Rating Scale:
Treating physician: 1.23 vs 0.49 [Belotero Balance (+) vs bovine collagen; P<0.001]
Blinded evaluator: 1.08 vs 0.5 [Belotero Balance (+) vs bovine collagen; P<0.001]
LEARN MORE ABOUT HOW TO USE BELOTERO BALANCE (+)
Belotero Balance (+) may be an ideal option for patients who want to address moderate to severe etched-in lines and wrinkles, who are new to fillers, or who want an alternative to laser treatments and chemical peels. Learn more about treatment areas, and see before-&-after images of real patients treated with Belotero Balance (+).
See Treatment Areas